对OJIP和MR820曲线的比较深入解析五种光合抑制剂的生理作用机制
2020-10-23 来源:www.hanshatech.com 点击次数:6387欢迎关注「汉莎科学仪器」微信公众号!
DOI:https://doi.org/10.1016/j.plaphy.2020.08.044
|
文章亮点:
|
|
文章结论:
|

本研究同时比较了TeA、DCMU、Bentazone、DBMIB和MV五种光合抑制剂(除草剂)对紫茎泽兰(Ageratina adenophora)瞬时荧光和MR820信号的影响,以期阐明它们对紫茎泽兰光系统的精确影响。
1. 五种抑制剂对叶绿素荧光OJIP曲线的影响
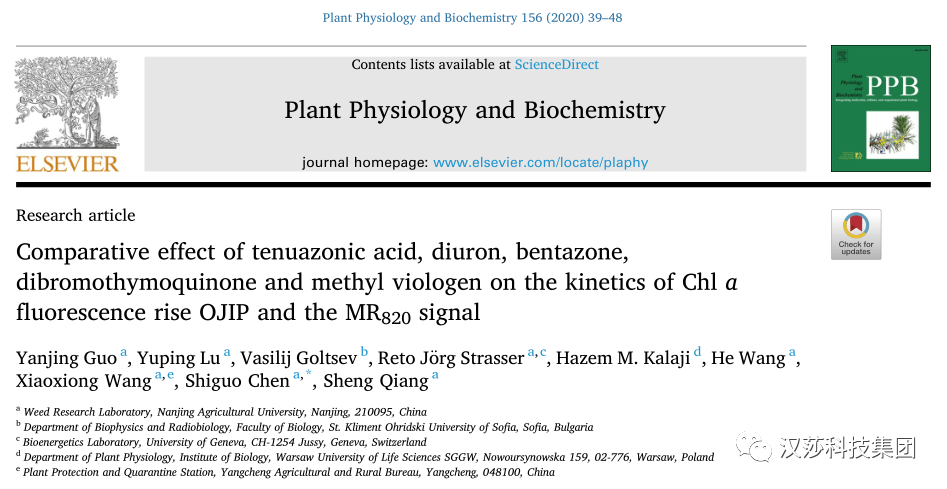
众所周知,DCMU是一种优秀的经典PSII除草剂,而TeA与DCMU相比是一种弱PSII抑制剂(Chen et al., 2007)。TeA、DCMU和Bentazone通过与D1蛋白相互作用影响初级光化学并阻断从QA 到次级醌受体QB 的电子传输(Nimbal et al., 1996; Bagchi et al., 2003; Strasser et al., 2004; Chen et al., 2007)。如上图,此三种抑制剂均增加了OJIP曲线的J-相,DCMU和Bentazone导致J-相快速增加接近于对照组P-相高度,并且导致I-P相消失;而TeA处理后J-相也明显增加,但仍存在I-P相。
Bentazone抑制光合电子传递的机制尚未明确,仅提出假说可与PSII的还原位点结合(Bagchi et al., 2003)。本研究结果表明Bentazone处理后J-相快速升高至P-相,表明其主要抑制QA以外的电子传递链,PSII活性反应中心RCs闭合速率快速上升,QA被迅速还原,QA-快速积累。
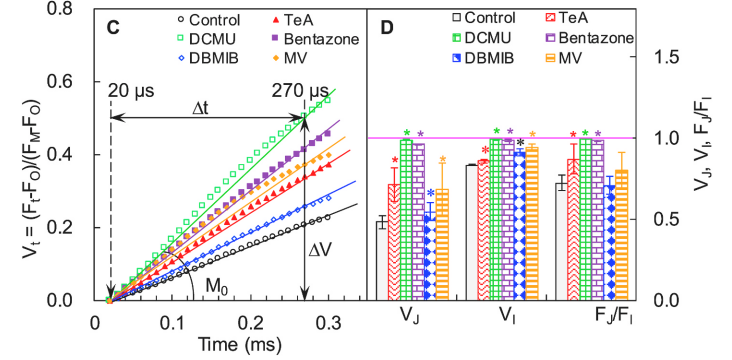
如上图C中DCMU、Bentazone和TeA处理后M0均高于对照组,进一步证明该三种抑制剂阻断了QA以外的PSII电子传输。
2. L-brand
|
L-band is known as an indicator of the grouping of the PSII units or energetic connectivity between antenna and PSII RCs (Srivastava et al., 1997; Strasser et al., 2004). |
L-峰可指示PSII不同组分间的聚集性或天线色素与PSII活性反应中心RCs的能量传递连通性。
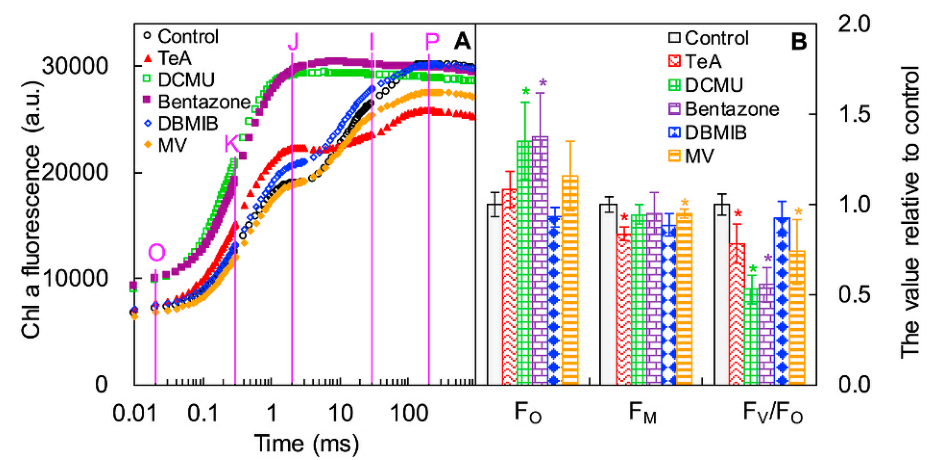
查看L-brand情况,如下图A中上部所示,对O点(20μs)和K点(300μs)之间进行标准化处理Wok = (Ft − Fo)/(Fk − Fo) 即可。同时以线性时间轴分析L-brand差分动力学(下图A底部),即 :
ΔWok = Wok(treatment or control) − Wok(control)
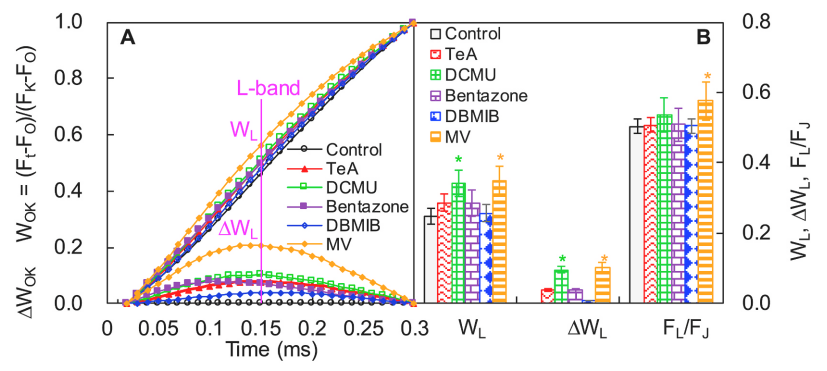
MV使L-brand增加最为显著,其它四种抑制剂均使L-brand略有增加。其中MV和DCMU处理样品的WL和ΔWL值显著升高,但DCMU处理样品的FL/FJ无显著性变化,表明DCMU处理引起的L-brand的升高是由于J-相升高引起的,而MV则降低了PSII不同组分间的聚集性或能量连通性而导致L-brand的出现。
3. K-brand
|
An increase of the K-step indicates the inactivation of OEC centers at the PSII donor side(Srivastava and Strasser, 1995; Srivastava et al., 1997; Strasser et al., 2004) |
| The occurrence of K-step has been observed in plants that suffer from heat or drought stress and is an indicator of the destruction of OEC(Strasser et al., 2004; Tόth et al., 2011; Chen et al., 2016). |
K峰的出现表明PSII供体侧的放氧复合体OEC(oxygen-evolving-complex)的失活。在遭受高温或干旱胁迫的植物中常观察到K峰的出现,是OEC受破坏的指示参数。
查看K-brand情况,如下图C中上部所示,对O点(20μs)和J点(2ms)之间进行标准化处理WOJ = (Ft − FO)/(FJ − FO) 即可。同时以线性时间轴分析K-brand差分动力学(下图C底部),即:
ΔWOJ = WOJ(treatment or control) − WOJ(control) 。
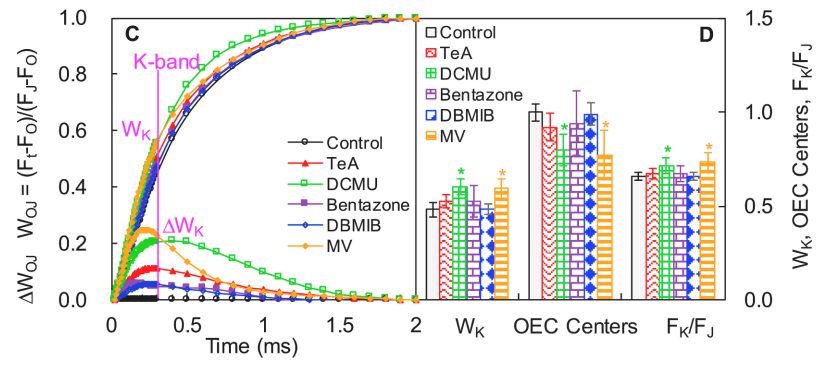
五种抑制剂均可引起K-brand的出现,而只有DCMU和MV显著改变了WK, OEC centers和FK/FJ,表明仅DCMU和MV确实损伤了OEC放氧复合体。
4. I~P相 & δRo
|
The maximal amplitude of WOI≥ 1 reflects the size of the pool of the end electron acceptors at the PSI acceptor side and the WIP represents the reduction rate of the end electron acceptor in PSI(Oukarroum et al., 2009; Yusuf et al., 2010). |
WOI≥ 1部分I~P相的振幅反应了PSI受体侧末端电子受体库的大小,WIP则反应了PSI受体侧末端电子受体的还原速率。
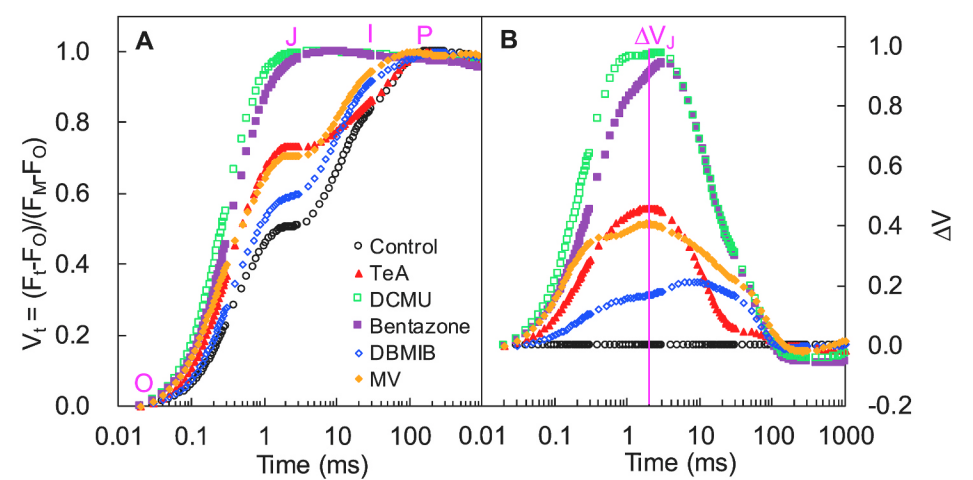
通过对O点(20μs)和I点(30ms)之间进行标准化处理WOI = (Ft − FO)/(FI − FO) ,WOI<1部分可观察J相变化,WOI≥1部分I~P相的振幅大小指示PSI受体侧末端电子受体库的大小。I~P相振幅越小,则表明PSI受体侧末端电子受体库所受抑制越强。
WIP = 0.5(上升曲线达到半值)处所需时间点可用来反应PSI末端电子受体库的还原速率。
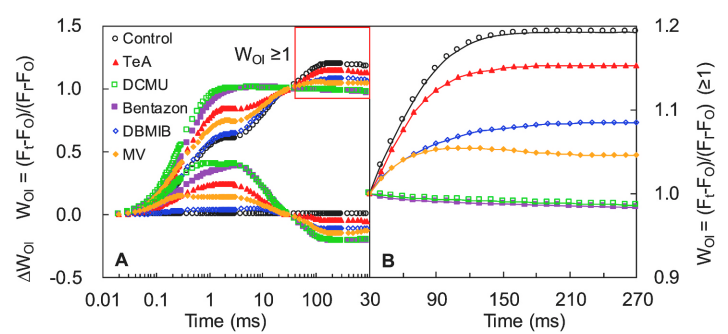
如上图A\B及下图C所示,DCMU和Bentazone处理使I~P相振幅完全消失、曲线上升半程时间升高,表明此两种抑制剂可抑制PSI受体侧末端电子受体库,且降低其还原速率。而TeA仅降低了受体侧末端电子受体库,对其还原速率不产生影响。
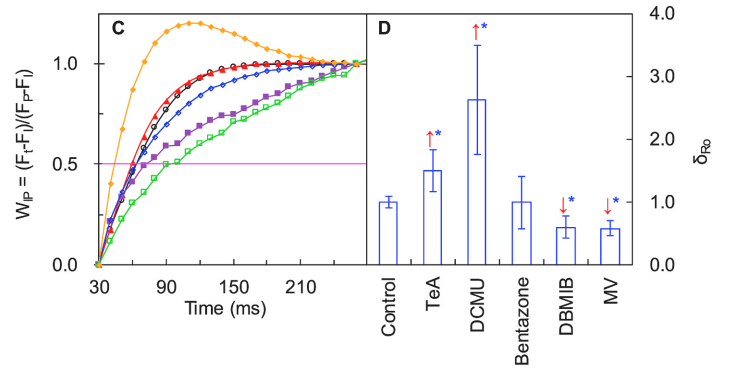
δRo = TR0/ET0,表示电子从还原的PSII和PSI系统间电子传递链的电子受体传输到PSI末端电子受体的概率。
上图D结果表明,TeA和DCMU显著提高了δRo,DBMIB和MV则大幅度降低了δRo,说明TeA和DCMU对PSI活性有一定的刺激作用,而DBMIB和MV对其有一定的抑制作用。
5. 820nm调制反射动力学曲线模型&参数
典型的820nm调制反射瞬态动力学曲线包括从MR0(约0.7ms)到MRmin(约7ms)的快速下降阶段(快相),然后是从MRmin到MRmax(约300ms)的缓慢增加相位(慢相)。
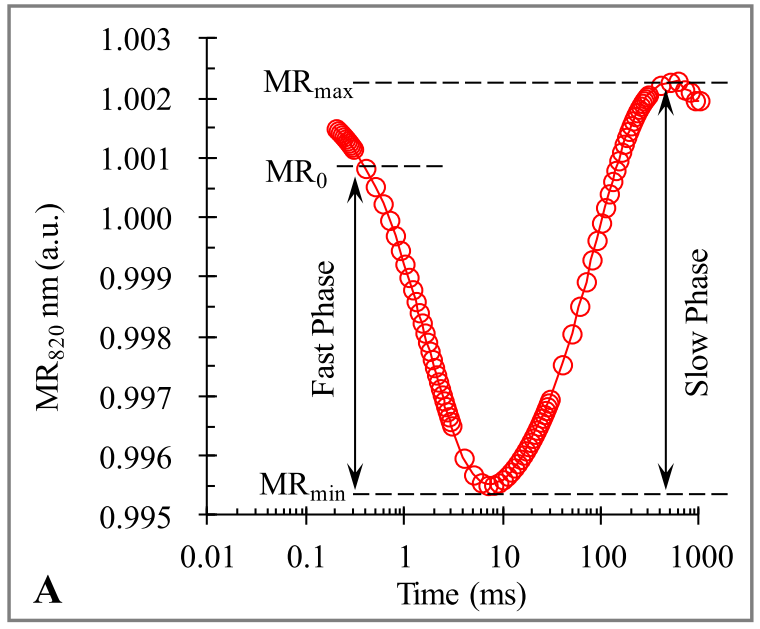
快相反映了PC和P700的氧化状态,可以量化为∆MRfast/MR0。慢相代表PC+和P700+的再还原状态,表示为∆MRslow/MR0。
**点MRmin时,PC和P700的氧化速率与还原速率达到平衡状态,氧化还原速率相等。
Gao等人2014年**提出由MR820曲线导出的两个参数Vox 和Vred,分别代表PC和P700的氧化和再还原速率,可以利用MR信号在两个特定时间范围内线性回归的初始斜率的绝对值来计算,分别为0.7~3 ms(快速阶段)和7~300 ms(慢速阶段)(Gao et al., 2014)。
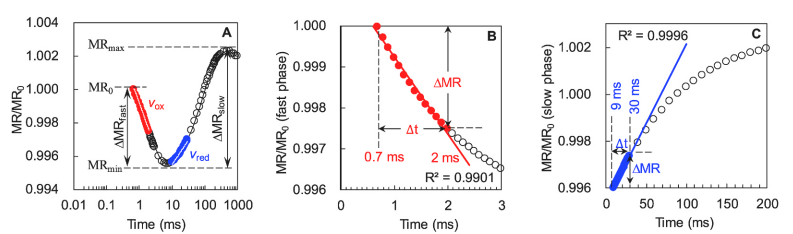
然而,这两个特定时间范围的MR/MR0信号与线性时间标度不是一条直线。因此在计算Vox和Vred时,如上图本研究作者创新性地提出了两个新的时间范围:0.7~2ms(Vox)和9~30ms(Vred)对其进行优化:
Vox =∆MR/∆t =(MR2ms – MR0.7ms)/(1.3 ms);
Vred =∆MR/∆t =(MR30ms – MR9ms)/(21 ms);

为了进一步证实五种抑制剂是如何影响PSI活性的,在M-PEA多功能植物效率分析仪(英国Hansatech)测定快速叶绿素荧光动力学OJIP曲线的同时,测定了820nm的透射动力学曲线。测定程序如下:1s饱和红光脉冲+10s远红光脉冲+第二次1s饱和红光脉冲。
两个红光脉冲被设置来诱导PC和P700的快速氧化(快相),**饱和红光脉冲后期P700随后被来自PSII的电子传输重新还原(慢相)。中间10s远红光脉冲被设计用来对PC和P700进行二次氧化。
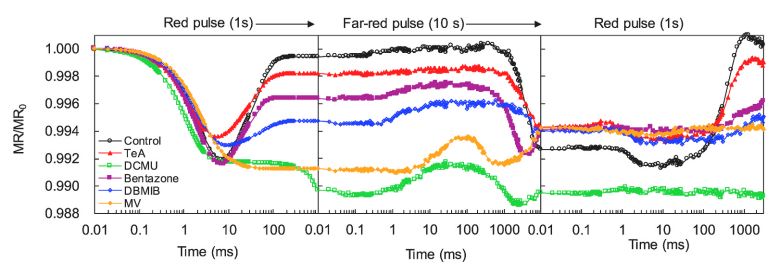
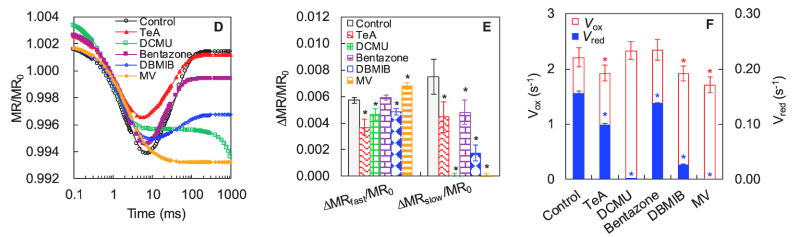
结果表明,DCMU、MV、Bentazone和TeA主要影响MR820信号的慢相部分。经DCMU和MV处理的样品在MR820信号动力学中慢相部分完全消失。而Bentazone和TeA仅部分影响MR820信号的慢相。
DCMU是一种优良的光合除草剂,通过与D1蛋白结合来阻断QA以外的电子传递。在DCMU的存在下,几乎没有电子可以通过QA来还原P700+和PC+
(Strasser et al., 2004)。MV直接从电子传输链捕获电子,速度几乎与PSII将其泵入链中的速率相同(Schansker et al., 2005)。在DCMU和MV处理的叶片中,没有电子参与P700+和PC+的再还原,而Bentazone和TeA不能完全阻断通过QA到PC+和P700+的PSII电子流。
DBMIB是一种人工醌,通过与细胞色素(cyt)b6f复合位点结合来抑制光合作用中的电子传递并阻止PQ库的再氧化(Belatik et al., 2013)。因此观察到DBMIB低了∆MRfast/MR0和∆MRslow/MR0的值以及Vox和Vred的值,这表明DBMIB分子既影响PC和P700的氧化又会对PC+和P700+的再还原产生影响。
6. 结论&创新性
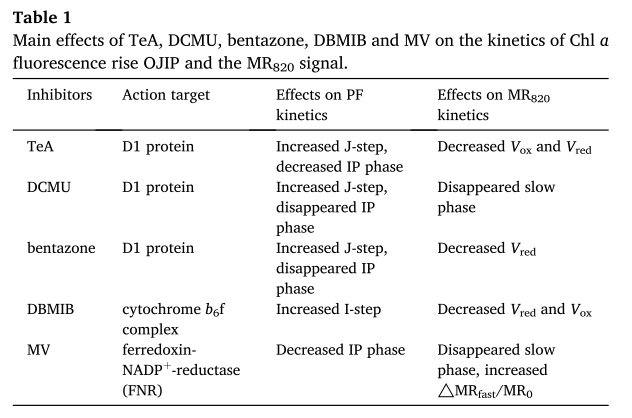
可见,叶绿素快速荧光动力学OJIP曲线和MR820动力学曲线为评价和监测PSII和PSI活性的变化提供了方便的方法和新的思路。这两种测量方法都清楚地显示了植物遭受不同光合抑制剂胁迫时的敏感性和优势。
另一方面,由于植物光合器官在每种抑制剂下的反应是特定的(表1),我们认为这种类型的研究可以帮助发现在植物生长期间使用了哪些除草剂,而无需进行化学分析。
这一发现有助于“plant talk”和“machine learning”领域,利用叶绿素荧光测量来确定植物的胁迫类型(Goltsev et al., 2012; Kalaji et al., 2018)。

参考文献:
-
Bagchi, S.N., Pistorius, E.K., Michel, K.P., 2003. A Synechococcus sp. PCC 7942 mutant with a higher tolerance towards bentazone. Photosynth. Res. 75, 171–182.
-
Belatik, A., Joly, D., Hotchandani, S., Carpentier, R., 2013.Re-evaluation of the side effects of cytochrome b6f inhibitor dibromothymoquinone on photosystem II excitation and electron transfer. Photosynth. Res. 117, 489–496.
-
Chen, S., Xu, X., Dai, X., Yang, C., Qiang, S., 2007.Identification of tenuazonic acid as a novel type of natural photosystem II inhibitor binding in QB-site of Chlamydomonas reinhardtii. Biochim. Biophys.Acta 1767, 306–318.
-
Chen, S., Yang, J., Zhang, M., Strasser, R.J., Qiang, S., 2016.Classification and characteristics of heat tolerance in Ageratina adenophorapopulations using fast chlorophyll a fluorescence rise O-J-I-P. Environ.Exp. Bot. 122, 126–140.
-
Gao, J., Li, P., Ma, F., Goltsev, V., 2014. Photosynthetic performance during leaf expansion in Malus micromalus probed by chlorophyll a fluorescence and modulated 820 nm reflection. J. Photochem. Photobiol., B 137,144–150.
-
Goltsev, V., Zaharieva, I., Chernev, P., Kouzmanova, M., Kalaji,H.M., Yordanov, I., Krasteva, V., Alexandrov, V., Stefanov, D., Allakhverdiev,S.I., Strasser, R.J., 2012. Drought-induced modifications of photosynthetic electron transport in intact leaves: analysis and use of neural networks as a tool for a rapidnon-invasive estimation. Biochim. Biophys. Acta 1817, 1490–1498.
-
Kalaji, H.M., Bąba, W., Gediga, K., Goltsev, V., Samborska,I.A., Cetner, M.D., Dimitrova, S., Piszcz, U., Bielecki, K., Karmowska, K.,Dankov, K., Kompała-Bąba, A., 2018. Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth. Res. 136, 329–343.
-
Nimbal, C.I., Yerkes, C.N., Weston, L.A., Weller, S.C., 1996.Herbicidal activity and site of action of the natural product sorgoleone.Pestic. Biochem. Physiol. 54 (1), 73–83.
-
Oukarroum, A., Schansker, G., Strasser, R.J., 2009. Drought stress effects on photosystem I content and photosystem II thermo tolerance analyzed using Chl a fluorescence kinetics in barley varieties differingin their drought tolerance. Physiol. Plantarum 137, 188–199.
-
Schansker, G., Toth, S.Z., Strasser, R.J., 2005. Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochim. Biophys. Acta 1706,250–261.
-
Srivastava, A., Strasser, R.J., 1995. How do land plants respond to stress temperature and stress light? Archs. Sci. Gen` eve 48, 135–146.
-
Srivastava, A., Guiss′ e, B., Greppin, H., Strasser, R.J., 1997.Regulation of antenna structure and electron transport in photosystem II of Pisum sativum under elevated temperature probed by the fast polyphasicchlorophyll a fluorescence transient: OKJIP. Biochim. Biophys. Acta 1320, 95–106.
-
Strasser, R.J.,Tsimilli-Michael, M., Srivastava, A., 2004. Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou, G.C., Govindjee (Eds.), Chlorophyll Fluorescence: A Signature of Photosynthesis. Kluwer Academic Publishers Press, Netherlands,pp. 321–362.
-
Tόth, S.Z., Nagy, V., Puthur, J.T., Kov′ acs, L., Garab, G.,2011. The physiological role of ascorbate as photosystem II electron donor: protection against photoinactivation in heat-stressed leaves. Plant Physiol.156, 382–392.
-
Yusuf, M.A., Kumar, D., Rajwanshi, R., Strasser, R.J.,Tsimilli-Michael, M., Govindjee, Sarin, N.B., 2010. Overexpression of γ-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: physiological and chlorophyll a fluorescencemeasurements. Biochim. Biophys. Acta 1797, 1428–1438.

